Note: this summary is focused on the new objective to study SPR and its equilibrium method for determining K_D
SPR-specific Assumptions based on its derivation
INVALIDATED
Sourced from Old SPR Paper with Derivations.pdf
Let [A] be Analyte concentration Let [L] be Ligand concentration
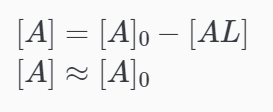
- the analyte concentration (flow solution) is considered to be in excess, and by extension, the complex formed on the sensor is negligible
- Knowing that the maximum complex concentration is equal to the concentration of ligand on the sensor surface,
- The concentration of analyte is maintained to be constant throughout the continuous flow setup of SPR
Clarification of the term “equilibrium”
From 0_BiacoreAssayHandbook-1.pdf
- The term equilibrium and steady state is used interchangeably
- The document defines steady-state to be conditions in which the net rate of complex formation is zero, suggesting that the term “equilibrium” also means the same thing
- Although, steady-state would be the better term to describe the conditions created by SPR because “equilibrium” may be mistaken with the thermodynamic equilibrium in which a close system is required
Clarifying the experimental process of SPR, specifically choosing the right ligand concentration to immobilize on the sensor
- the equipment must be equilibrated based on the injected flow buffer - if any form of drift is detected, it must be corrected through cleaning of the sensor/system
- A “Max additional response ()” must be picked, generally in the 50 to 150 RU range as defined in SPR techniques.pdf with the following formula:
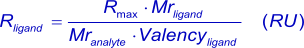
- Based on the calculated ligand must be immobilized on the sensor until the baseline signal equals
Where is steady-state and kinetics analysis used
From 0_BiacoreAssayHandbook-1.pdf
-
suggests that kinetics and steady-state analysis are rarely conducted together for a certain analyte-ligand pair - defines common SPR practices
-
Suggests the following criteria in which either kinetics or steady state must be used
(Where contact time is the duration for which analyte flows over the sensor surface) (Where injected volume refers to the total volume of analyte solution delivered over the sensor surface during the association phase)
-
Low contact time, and thus low injection volume of analyte or high flow rate
- In such a scenario, steady-state may not be reliably reached, and the kinetic data must be used
-
High contact time, and thus high injection volume of analyte
- In such a scenario, it may be hard to assess the rapid association curve for an accurate analysis, which is why the equilibrium method may be used
Specifics of the Steady-State SPR Method
From SPR techniques.pdf
Suggests that equilibrium measurements of analyte and ligand affinity are unaffected by mass-transport or rebinding artifacts
-
to justify, considering that equilibrium determination methods simply require the signal produced at steady-state, whether the association time period is influenced by the diffusion rate of the analyte will not affect the signal at steady-state = the progress curve is not being analyzed with this method
-
Advantages
- The paper suggests that the equilibrium, steady-state method allows for the use of high analyte concentrations because the progress curve of analyte-ligand binding is not assessed
- The equilibrium method can be applied for small analytes because the SPR sensors measure the mass of material binding the sensor surface. More immobilized ligand is required for the binding of small analytes to produce good signals. High ligand density on the sensor prevents kinetic analysis, and only allows equilibrium analysis
Suggests that injection time for the equilibrium/steady-state method can be assessed based on the value “The time it takes to reach equilibrium is determined primarily by the dissociation rate constant or koff; a useful rule of thumb is that an interaction should reach 99% of the equilibrium level within 4.6/koff seconds.”
- For high affinity interactions (K_D<10 nM), slow koff values relative to kon suggests that longer time is needed, which is inconvenient, which is why kinetics is used
Future Plans
- Confirm the claim that equilibrium methods are unaffected by mass transport or rebinding artifacts
- Do rebinding artifacts contribute to drift?
- Continued research on equilibrium-method specifics for SPR